A major pharmaceutical company, with multiple facilities in its global manufacturing network, approached Matcon for a pharmaceutical powder handling solution that could minimize the risk of segregation during the production process.
The pharmaceutical company has a growing portfolio of over 7,000 products, which includes common branded and generic prescription drugs and over-the-counter remedies, along with Active Pharmaceutical Ingredients (APIs).
About the Application
The new drug, levothyroxine - used to treat hypothyroidism, is made via a geometric dilution mixing process and presented in a tablet form. The product has an API content as low as 0.02% to 0.05% so the company needed to ensure that the blend uniformity (BU) was in the range of 95% to 104% and content uniformity (CU) stayed within set limits during processing.
With their rectangular design & blending geometry within the Matcon Blender, Matcon IBCs and their unique 'Cone Valve' technology ensures consistent and maintained CU, even during the transfer & feed of the IBC contents to the Tablet Press.
In addition to these niche requirements, the customer also wanted to maintain Occupational Exposure Band (OEB) Level 3 by using dust-tight connections. Together with our passive and active valve, and having the right seals in place at various intersections of the powder transfer process, ensures that our dust tight connections achieve OEB Level 3.
During the initial product development stage, Matcon was able to supply a blending system to manufacture ‘pilot’ batches which were used in filing the new drug application (ANDA) for US FDA approval. A production-scale powder handling IBC system solution followed once the US FDA authorization was received.
What did the customer want to improve?
Before Matcon was contacted, the company was finding that with their original equipment the blended powder materials, that made up this product, were prone to segregation, especially when being transferred between the manufacturing process stages of milling (particle size reduction), blending and compression. This blend segregation led to poor product integrity and therefore wasted high value product.
To meet the rapidly changing health needs of the global population, the company needed to minimize any threat of product segregation to ensure a consistent high-quality supply of pharmaceutical products.
The pharmaceutical company identified that Matcon Cone Valve technology would remove the risk of blend segregation and meet their needs to provide a high level of containment through the processing stages.
How does Powder Segregation occur?
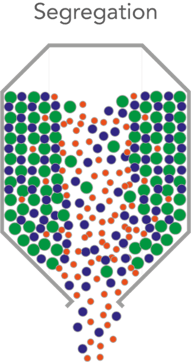
Segregation occurs when particles separate due to differences in their size, shape, or density. It can be caused by the way in which a powder blend is handled throughout the production process.
If dry granulation using roll compaction is a process step in your pharmaceutical manufacture, it is important to prevent material from segregating as the quality of the granules produced are dependent upon the quality of the in-feed from the IBC.
It is well known in the industry that butterfly valve and slide valve IBCs can cause material segregation as they create core or funnel flow during the discharge process (as shown above).
The flow of material only occurs in a narrow vertical channel from the open area of the outlet to the top surface of the material in the container, creating a hole within the vessel, also known as ‘rat-holing’. As the material on the top always prefers to flow towards the central core, it results in material being held along the sides of the vessel, as the powder moves to the central core it enables particles to roll and separate creating segregation.
The Implications of Segregation Are Critical.
If the material de-mixes or segregates as it is being fed into the tablet press, it will result in the incorrect proportions of the active ingredient in each tablet – and result in batches having to be scrapped. Due to the formulation of this levothyroxine product, it must guarantee to have the correct ratio of active and excipient within the tablet and therefore if production quality cannot be guaranteed, it will lead to issues with the regulatory authorities.
How Matcon’s IBC solution can eliminate powder blend segregation
At the heart of the Matcon IBC system is our unique Cone Valve that has been used in the Pharmaceutical industry for over 35 years. Our IBCs prevent the problem of powder blend segregation by promoting mass-flow discharge, where all the powder particles in the container flow downward at the same rate across the whole cross-sectional area of the container, creating uniform flow. Material prefers to flow down the vessel walls rather than the core, consequently there is no movement of individual particles across each other, or rolling or dead zones – hence no segregation.
This also prevents material being left behind on the walls of the IBC – no stagnant material, no rat-holing and a first in-first out (FIFO) sequence of material movement.
See the Matcon Cone Valve IBC in action below.
Feed to the downstream Wet Granulator and Tablet Press via the Matcon Discharge Station is both controlled and automated, ensuring a consistent feed of finished product which is of a high quality and blend homogeneity, which will ultimately help meet the regulatory requirements.
Flow can be shut off during discharge without damage to the material.
Key Benefits of Cone Valve Technology
- Guaranteed and controlled powder flow
- Prevent segregation of mixed materials
- Dust-free transfer of materials
- Accurate, automated discharge procedure
- Complete discharge for high yields
- Contained solution for product integrity
What equipment did Matcon install?
The new Matcon IBC system was implemented as part of the expansion of an existing facility at the pharmaceutical manufacturer's India operation. The installation included the following process equipment:
- 1000L and 1500L stainless steel Cone Valve IBCs for collection and movement of materials in various stages such as Dispensing, Sifting with The Quadro® FlexSift, Milling, Blending, Granulation and Compression
- Matcon IBC Blender accommodating IBCs from 1000L to 2000L
- Pillar lift mounted Discharge System for Feeding from IBCs to Granulation and Compression Machinery
How Matcon Helps Pharmaceutical Manufacturers
A further vital aspect to achieving a successful installation of an IBC system lies with the experience of how to apply the technology into the various processing steps and connect it to any existing pharmaceutical equipment.
This is where the Matcon team offers great value – we strive to supply you with a system that brings you the maximum profitability and product quality. We aren’t just selling IBCs as a commodity, we take a full look at what your manufacturing needs are and design a system that handles your batches in the right quantities, optimizes the use of processing equipment and reduces the amount of manual handling needed to produce your product. We help you to make the right investment.
Posted by
Matt Baumber
